Project activity overview
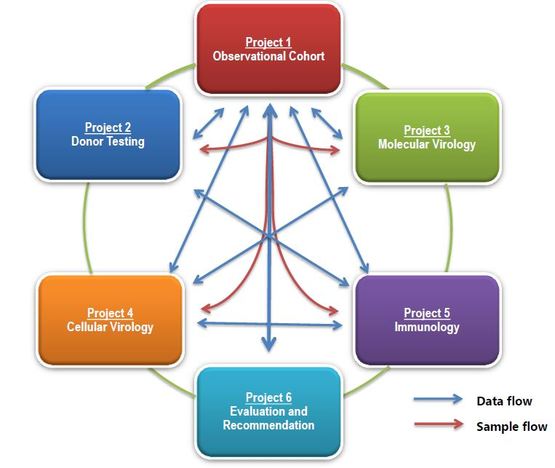
Project 1. Observational cohort ( A. Wensing / G. Hutter)
This project comprises the observational cohort in HIV-positive patients receiving allogeneic SCT. The major objectives are (i) guidance in the process of ethical board approval for additional sampling and research; (ii) provision of sampling protocols, organization of webbased data-submission and sample flow; (iii) clinical consultation on the SCT procedures considering hematological issues, HIV-related aspects and clinical virology and interpretation of co-receptor tropism; (iv) dissemination of the consortium activities to key stakeholders
This project addresses the typing of hematopoietic stem cells (HSC) for the presence of the CCR5-Δ32/Δ32 from suitable donors. The current criteria of donor choice follows the policies and strategies of each transplant center for search of an HLA matched HSC donor. In case of cord blood grafts the criteria will be based on number of HLA disparities and cell dose. The major aims of this project are (i) to organize screening of potential donors for the CCR5Δ32 allele; and (ii) to facilitate contact with international donor registries and cord blood banks to identify suitable donors with CCR5Δ32 allele.
Project 3. Molecular virology (M. Nijhuis)
This project addresses in depth molecular characterization of the size, genetic structure and co-receptor usage of the viral population in the peripheral blood. The major objectives include: (i) genotypic characterization of viral co-receptor usage, (ii) longitudinal determination of the size and dynamics of the viral reservoir, including different molecular HIV-1 species in different T-cell subpopulations, (iii) longitudinal assessment of the genetic structure of the viral reservoir, trough the phylogenetic analysis of the gp120-V3-loop and Gag.
Project 4. Cellular virology (J. Martinez-Picado)
This project pursues (i) to rescue and quantify replication competent virus from CD4+ T cells from blood and tissues (when possible), and (ii) construct Env-recombinant viruses to their (iii) functional characterization (co-receptor phenotypic tropism and replication capacity), and their (iv) molecular comparison with sequences in project 3. Single-copy assay from plasma and CSF will also complement molecular virology determination in project 3. Finally, (v) reconstituted CD4+ T cells after engraftment are challenged ex vivo with R5-and X4-tropic viruses.
Project 5. Innate and adaptive (T/B-cell) immunity (A. Saez-Cirión)
This projects explores different levels of immune responses in the patients, by assessing (i) the co-receptor expression of CCR5 and CXCR4, (ii) the expression of KIR/HLA complexes on NK cells, (iii) T-cell activation and proliferation capacity, (iv) to HIV-1 specific T-cell responses and regulation Treg, and finally (v) B-cell induction of HIV-1-specific antibodies (IgG).
Project 6. Evaluation and
recommendations (G. Hutter)
This project covers the evaluation of the transplantation outcome.
The objectives are (i) to formulate a clinical guidance
document for allogeneic SCT in
HIV-1-infected individuals with hematological malignancies; and (ii) to formulate a progress document on
restrictions and conditions for interruption of
cART after allogeneic stem cell
transplantation.
Observational cohort
Altogether we study allogeneic SCT recipients with HIV-1 infection, collecting complete information on underlying malignancy, chemotherapy, transplant procedure, donor selection, HIV-tropism, cART, and a variety of samples taken before and after the transplant.
To systematically study recipient samples for:
- multiple complementary quantifications of the viral reservoirs
- molecular and functional characterization of infecting virus
- immunological determinations during the multiple phases of an allogeneic SCT, which might help to understand the biological bases of a potential new case of HIV-1 cure.
Clinicial Guidance
In addition, hematologist or infectious disease specialists who would
like to receive clinical guidance on an allogeneic SCT procedure can
contact the clinical consultation team of the IciStem project via the IciStem coordination center.
Hematology:
J. Diez-Martin, Department of Hematology and Hematopoietic transplantation, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
G. Hütter, CellexGmbH, Dresden, Germany.
J. Kuball, Department of Hematology, University Medical Center Utrecht, Utrecht, the Netherlands.
V. Rocha, Department of Clinical Hematology, Oxford University Hospitals NHS Trust, Oxford Cancer and Haematology Centre, Churchill Hospital, Oxford, United Kingdom.
Infectious Disease:
J. Schulze zur Wiesch, Infectious Diseases Unit, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Clinical Virology:
A. Wensing, Department Medical Microbiology, University Medical Center Utrecht, Utrecht, The Netherlands.
Clinical work group
E. Steel, MD, Hematologist, Ghent University, Belgium
L. Vandekerckhove, Infectious Disease Specialist, Ghent University, Belgium
P. Ellerbroek, Infectious Disease Specialist, University Medical Center Utrecht, the Netherlands